Conduction and Convection are two out of three methods of heat transfer. The significant difference between conduction and convection lies in the way the transfer of heat takes place. The third mode of heat transfer is radiation however, conduction and convection are regarded as quite dominant in various practical applications.
In the process of conduction, the heat is transferred when there exists a direct contact between the surface to be heated and the source of heat. As against, in the process of convection, the heat transfer takes place through indirect contact.
In this content, we will see on what factors conduction is differentiated from convection. Let us have a brief idea about what is Heat?
So, basically, heat is regarded as a form of energy that can be transferred from one region to compensate for the difference in temperature.
Content: Conduction Vs Convection
Comparison Chart
| Basis for Comparison | Conduction | Convection |
|---|---|---|
| Basic | This mode of heat transfer requires direct connection between the two bodies. | This mode of heat transfer not needs direct connection between the regions where heat transfer is taking place. |
| Noticed in | Generally Solids | Generally Fluids and Gases |
| Arises from | Molecules at rest or free electrons | Molecules in motion |
| Necessity | Direct contact | No direct contact but an intermediary is required. |
| Cause of occurrence | Temperature difference | Difference in density |
| How it takes place? | Due to molecular collision. | Due to diffusion of heated particles. |
| Speed of heat transfer | Slow | Comparatively fast |
| Example | Heating of a metallic rod when placed in high heat. | Heating of liquid within a vessel which is placed at high flame. |
Definition of Conduction
Conduction refers to a process of transfer of heat energy from one surface to another which are in direct contact with each other. In the mechanism of conduction, the heat transfer takes place in a stationary medium i.e., solids. Fourier’s law explains the rate of heat transfer while conduction.
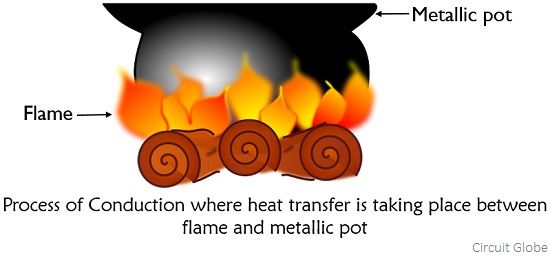
The figure below shows the pictorial representation of how heat transferring takes place during the process of conduction:
How the process of conduction takes place?
The process of conduction occurs in a way that here the heat energy is transferred when collision occurs between adjacent molecules vibrating with high velocity. These high-velocity molecules when come in direct contact with the molecules of solids which are at room temperature then the molecular vibration is transferred at the point of contact. Further, these vibrating molecules transfer the vibration to their adjacent molecule and in this way the body at room temperature gets hot.
Just like the molecular vibration in solids, conduction takes place in a similar way in liquids and gases as well. However, due to less molecular density in liquid and gas, the transfer of energy does not take place like that of solids as conduction is the transfer of energy between two bodies that are in direct contact.
Definition of Convection
The mode of transfer of heat by the displacement of fluid molecules from one region to another is known as Convection. Convection is generally of two types one is forced while the other is natural.
Natural convection is when the motion in a medium occurs due to the difference in temperature of two liquids when mixed. Whereas forced convection is the one in which motion is the result of any external unit like a pump or blower.
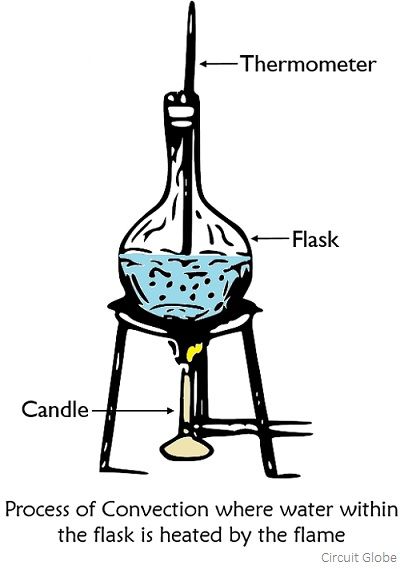
How convection takes place?
During convection, the transfer of heat takes place in a way that the molecules of fluids and gases after gaining enough energy become less dense and their increased buoyancy causes the molecules to rise up. While the molecules that are at low temperature has low buoyancy and thus falls down and get closer to the flame. Thus will get heated up and will exchange their positions with molecules of low temperature. In this way, the transfer of heat within the molecules of fluids or gases takes place.
Key Differences Between Conduction and Convection
- The key factor of differentiation between conduction and convection is that conduction occurs in solids i.e., material bodies. On the contrary, convection is generally noticed in fluids and gaseous substances.
- For the process of conduction to take place direct contact between the surfaces is required because only then the heat transfer will take place. While, for the process of convection to occur, no direct contact is necessary however, an intermediary is needed that will act as a carrier to take the heat from one region to another.
- Conduction occurs when there is a difference in temperature between two solid bodies that are in direct contact with each other. While convection occurs due to differences in the molecular density of the fluid.
- Conduction is the result of vibration between the closely packed molecules of a solid when the heat is provided. This molecular vibration of one surface is transferred to the nearby particles of another surface which is in direct contact. While convection is the result of a collision between less dense molecules of liquid and gas during motion. Here the molecules with high energy transfer their energy to the molecules with low energy and in this way particle energy is transferred.
- Conduction is a slow process as here a number of molecules in solids are more while convection takes place comparatively at a faster rate due to dispersed molecular orientation of liquid and gas.
- When a metallic rod is placed on a high flame then due to direct contact the vibration of molecules of the flame is transferred to the molecules of the metallic rod at the point of contact. This initially increases the temperature of the rod at the region which is in direct contact with the flame and after some point of time, the molecular vibration transfers the heat energy to the whole rod.
However, heating of a liquid which is placed on a high flame does not require direct contact with the flame but the vessel in which it is present act as an intermediary that transfers the molecular vibration of the flame to the liquid within the vessel.
Conclusion
Thus, the above discussion concludes that the two modes of heat transfer differentiated here explain the manner in which the transfer of heat takes place along with the material matter in which it is noticed.