The cell and battery both store the chemical energy and then transforms the stored chemical energy into an electrical energy. One of the major difference between the cell and the battery is that the cell is the single unit, whereas the battery is the group of cells. Some other differences between them are explained below in the comparison chart.
Content: Cell Vs Battery
Comparison Chart
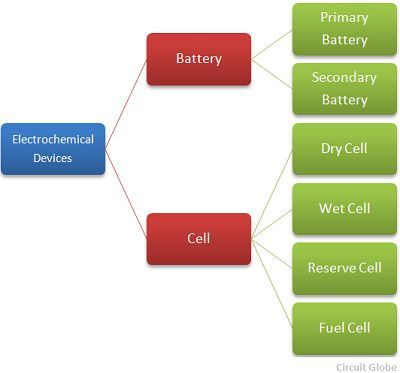
The classification of the electrochemical device is shown in the figure below.
Definition of Cell
The cell is a single power generating unit which stores the chemical energy and then converts it into electrical energy. It has two electrodes namely cathode and the anode.The cell has an electrolyte, a chemical substance that reacts with the electrodes and produces electric current.
The redox reaction occurs between the electrolyte and the electrodes and due to this reaction the electric current starts flowing through an external circuit. The cell is mainly classified into four types.They are the wet cell, dry cell, reserve cell and fuel cell.The wet cell uses a liquid electrolyte, and in the dry cell, the electrolyte is in the form of the powder.
Definition of Battery
The battery is a device which consists two or more units of an electrochemical cell.The positive terminal of the battery is known as the cathode whereas the negative terminal of the battery is known as the anode. The battery is of two types, i.e., the primary battery and the secondary battery.
The primary battery is irreversible or cannot be reused, whereas the secondary battery is rechargeable. In a primary cell, the chemical energy is inherently present, and in the secondary cell, the electrical energy is induced by the external source.
Key Difference Between Cell and Battery
- The cell is a single unit device which converts the electric energy into chemical energy, whereas the battery is the group of the cell.
- The cell is either dry, wet, reserve and fuel types depends on the types of electrolytes used, and the battery is either non-chargeable or rechargeable.
- The cell has a single unit, and hence it is light and compact whereas the battery is a combination of cells which increase the size of the battery and make it’s bulky.
- The cell supply power for a short time, whereas the battery supply power for the long duration.
- The cell is cheap as compared to the battery.
- The cell is mostly used in the clocks, lamp, etc. which requires less energy, whereas the battery is mostly used in the automobiles, inverter, etc.
The galvanic cell, Daniel cell, Leclanche cell are some of the examples of the cell while lead-acid battery, lithium-ion battery, magnesium ion battery, etc. are the types of the battery.



Thanks a lot for this article, it help me a lot to complete my research. I appreciate.
Great article.
It was quite informative
Thanks a lot for this comparison.this help me a lot
thanks
Interesting …..
Easy language. Really helped me. Thanks a lot. ❤❤
very educative thanks