Schottky and Frenkel Defects are the two crucial subcategories of point defect. The significant difference between Schottky and Frenkel Defect is that the Schottky defect arises when atoms (consisting of both cation and anion) are missing from the crystal lattice. As against, Frenkel defect arises when a smaller ion (in general cation) gets dislocated from its actual position to an interstitial site.
In Schottky defect, the combinational vacancy of cation and anion within the crystal lattice exists. As both cation and anion are missing thus, the overall electroneutrality of the crystal remains undisturbed. However, this is not the case with the Frenkel defect as here the smaller ion dislocated to another location within the crystal.
Before learning in detail the differences between the two let us first have a basic idea about
What is a defect?
Defect in solids, in general, corresponds to the irregularities in the constituent particles that form the crystalline structure. Generally, a missing atom or an impurity in place of the actual atom leads to a defect in the solid. You must note here that the physical and chemical behavior of the solids varies with the defect present in that particular solid material.
As we have discussed that Schottky and Frenkel defects are part of point defect. Basically, a point defect is an error at the atomic site in a crystal. Sometimes this can be in a form of a missing atom representing vacancy while sometimes atoms get displaced from the correct site to a location that generally remains unoccupied and this location is called an interstitial site.
Content: Schottky Vs Frenkel Defect
Comparison chart
| Basis for Comparison | Schottky Defect | Frenkel Defect |
|---|---|---|
| Basic | This arises when cations and anions are absent from their respective lattice sites. | This arises when cations leave their respective place in the lattice and moves to an interstitial site. |
| Defects associated | Vacancy defect | Vacancy defect at actual point and interstitial defect at the new location. |
| Generally seen in | Ionic solids having comparable cationic and anionic sizes. | Ionic solids having large difference in size of cation and anion. |
| Displacement | Both anion and cation move out from the crystal. | Here though displacement occurs but no ion displaces out of the crystal. |
| Crystalline density | Decreases | No effect |
| Produces | Two vacancies, one of cation and other of anion. | One vacancy and one interstitial site. |
| Example | NaCl, KCl, CsCl, etc. | Agbr, AgCl, ZnS, etc. |
Definition of Schottky Defect
The Schottky defect is a vacancy defect exhibited by ionic solids. In this defect, the ions of the solid (both cation and anion) leave their place vacant and move out from the crystal lattice. This creates vacancy within the crystalline structure. The figure below represents Schottky defect: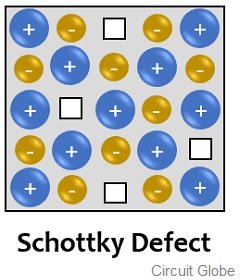
It is clear from the figure shown above that in the crystalline solid, at some points there is an ionic vacancy. You can clearly see that the ions that have left their places are not dislocated to any empty space within the lattice. This results in a decrease in the overall density of the material.
Definition of Frenkel Defect
Frenkel defect is sometimes called dislocation defect and is a combination of vacancy and interstitial defect. In this defect, cations leave their actual position within the lattice and occupy an empty space within the lattice, generally known as an interstitial site. The figure below shows the Frenkel defect: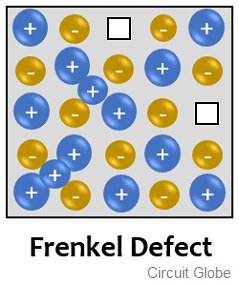
From the figure given above, we can say that here the cation has left its original position which is vacant and displaced to space that is not occupied by an ion within the crystal lattice itself.
Key Differences Between Schottky Defect and Frenkel Defect
- The key factor of differentiation between Schottky and Frenkel defect is related to the motion of ions within or out of the crystal lattice. The Schottky defect is the result of the motion of an atom out from the crystal lattice. While Frenkel defect is the result of displacement of ion from actual site to an empty space within the crystal lattice.
- As in the Schottky defect, the ions move out of the crystal thus, the overall crystalline density shows reduction due to this defect. However, in Frenkel defect, as the ions only displace to a new location but do not exit the crystal thus, its crystalline density remains the same.
- Schottky defect is prominent in those solids in which the difference in the size of cation and anion is not much. On the contrary, the Frenkel defect is shown by the solids that possess a large difference in the size of cation and anion.
- A Schottky defect shows two vacancies in the crystal structure of the solid where one vacancy corresponds to cation and the other corresponds to the anion. On the flip side, the Frenkel defect represents a single vacancy at the actual site of the ion as that ion takes another empty space in the crystal lattice.
- A well-known German Physicist Walter H Schottky explained Schottky defect. While Frenkel defect was proposed by a Soviet Physicist Yakov Frenkel.
- Crystalline solids like sodium chloride, potassium chloride, caesium chloride, etc. exhibit Schottky defect. While crystalline solids such as silver chloride, silver bromide, and Zinc sulphide, etc. display Frenkel defect.
Conclusion
The above discussion concludes that both Schottky and Frenkel defects are associated with crystalline solid and leads to cause vacancy at the actual site. But the difference is in Schottky defect the ions leave the crystal lattice completely but in Frenkel defect the ions take an interstitial site within the lattice.
A crystalline solid, silver bromide (AgBr) is the one that displays both Schottky and Frenkel defect.
