Electrolytes and non-electrolytes are the two chemical compounds that are classified according to their conductive and non-conductive nature through the aqueous solution. The significant difference between electrolytes and non-electrolytes is that electrolytes are the chemical compounds whose aqueous solution allows conduction. On the contrary, non-electrolytes are those chemical compounds whose aqueous solution is of non-conductive nature.
This article will provide you with the idea of how electrolytes are differentiated from non-electrolytes. But before that have a look at the contents inclusive in this article.
Content: Electrolytes Vs Nonelectrolytes
Comparison Chart
| Basis for Comparison | Electrolytes | Nonelectrolytes |
|---|---|---|
| Basic | These chemical compounds completely break down into ions. | These chemical compounds never completely break down into ions. |
| Nature | Conductive | Non-conductive |
| Type of bond | Ionic bond | Covalent bond |
| Composition | Acids, Bases and salts | Carbon containing compound, sugar |
| Compound type | Polar | Non-polar |
| Example | Sodium, potassium, magnesium, etc. | Glucose, ethanol, etc. |
Definition of Electrolytes
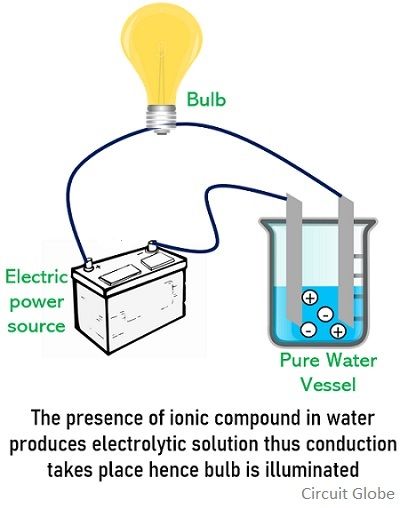
A substance which when dissolved in solvent produces a solution that is of electrically conducting nature is known as an electrolyte. Acids, bases, and salts are the most familiar electrolytes.
Generally, when these are dissolved in water then it gets completely ionized. This means the cations and anions are separated when the substance is dissolved and get dispersed properly throughout the solvent.
In a solution, it exists in an electrically neutral state but when a certain electric potential is applied then the cations present in the solution get attracted towards the negative electrode while the anions move towards the positive electrode. And this movement of cations and anions towards the respective electrode results in the flow of current thereby making it electrically conductive.
Electrolytes are really important for the proper functioning of the human body. Thus, it should be consumed in adequate amount. Also, in the human body, electrolytes are found in blood, sweat, and urine.
Electrolytes mainly maintain balancing within the internal environment of the body. Thus, are used in the functioning of the nervous system along with muscular functioning. Also, used to keep the body hydrated and maintaining a proper pH level. Hence, the imbalance of electrolytes in the body is not good for health and this may result in headache, fatigue, muscle weakness, numbness, irregular heartbeat, etc.
Definition of NonElectrolytes
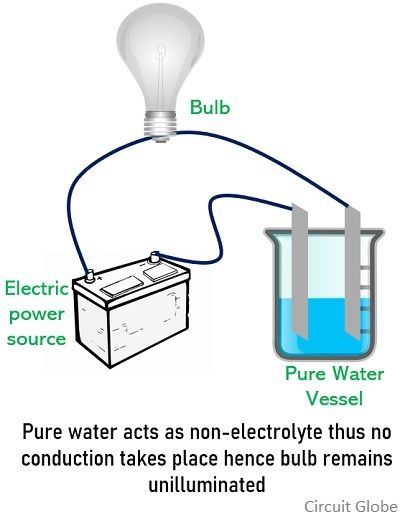
Nonelectrolytes are substances that never get completely ionized when dissolved in an aqueous solution. This implies nonelectrolytes fail to dissociate into ions when dissolved into a solvent. Thus, complete ionization does not occur when these substances are mixed with solvent hence these are unable to possess conductive nature.
Generally, water is known to be the most important ionizing solvent used to form electrolytic or non-electrolytic solutions. However, water is not the only solvent used for this purpose.
Unlike electrolytes, when nonelectrolytes are dissolved in aqueous solution then due to the presence of a covalent bond between the molecules, it does not break down into ions completely. Due to incomplete ionization, the cations and anions are not separated and hence even in the presence of externally applied potential, the conduction of electric current does not take place.
So, mainly the type of bond which is present between the molecular structure controls the dissociating of ions when present within the aqueous solution.
Sugar is a nonelectrolyte because a sugar solution does not permit electric current to flow through it.
Key Differences Between Electrolytes and Nonelectrolytes
- The key factor of differentiation between electrolytes and nonelectrolytes can be understood with the help of their names. Electrolytes act as electric conductors when present in a solution, on the contrary, nonelectrolytes never possess the behavioural characteristics of the conductor when present in a solution.
- Electrolytes when dissolved in a solvent get completely break down into ions i.e., separated as cations and anions. As against, nonelectrolytes when dissolved in a solvent never completely breakdown into ions.
- The bond that exists between the compounds that form electrolytes is ionic in nature. However, generally, nonelectrolytes have a covalent bond between the molecules.
- Due to the presence of both positive and negative charges (because the difference in electronegativity is between 0.3 to 1.4) in electrolytes, these are configured as polar compounds. While there is no significant difference in electronegativity between the molecules (difference in electronegativity here is generally 0 to 0.2) thus two different types of charge carriers do not exist, hence are configured as non-polar compounds.
- The compounds that show electrolytic behaviour are sodium, potassium, calcium, etc. While the non-electrolytic compounds are glucose, ethanol, etc.
Conclusion
Thus, from this discussion, we can conclude that these two substances are mainly classified according to their ability to conduct electric current or not. But at the same time, this property of the substance crucially shows dependency on the type of bond existing between the molecular structure of two types of substances.