The addition of impurities to a semiconductor material leads to cause variation in the conducting nature of the material. The fundamental factor of difference between donor and acceptor impurities is that a donor impurity donates charges to the semiconductor. As against an acceptor impurity accepts the charges from the semiconductor material.
Basically, the phenomenon of adding impurity to a semiconductor is known as doping. Doping contributes to the conductivity of the material. There are various factors of differentiation between donor and acceptor impurities which we will see in this content.
Content: Donor Vs Acceptor Impurities
Comparison Chart
| Basis for Comparison | Donor Impurities | Acceptor Impurities |
|---|---|---|
| Basic | The impurities that increases conductivity by donating charge is known as donor impurities. | Those impurities that accepts the charge for increasing conductivity is known as acceptor impurities. |
| Also referred as | pentavalent impurities | trivalent impurities |
| Number of valence electrons | 5 | 3 |
| Forms | n-type semiconductor | p-type semiconductor |
| Group position in periodic table | Group V | Group III |
| Examples | Phosphorus, Bismuth. | Aluminium, Boron. |
Definition of Donor Impurity
A dopant having 5 electrons in its valence shell when doped with a semiconductor to increase its conductivity is known as a donor impurity. It holds the ability to donate an extra electron present in its valence shell to the neighbouring atom. Thus is given the name ‘donor’. Due to the presence of excess negative charge, it forms the n-type region. Thus donor impurity is used to form n-type semiconductors.
Group V elements are said to be donor impurity because they consist of 5 electrons in the outermost shell. Thus it is also known as pentavalent impurity.
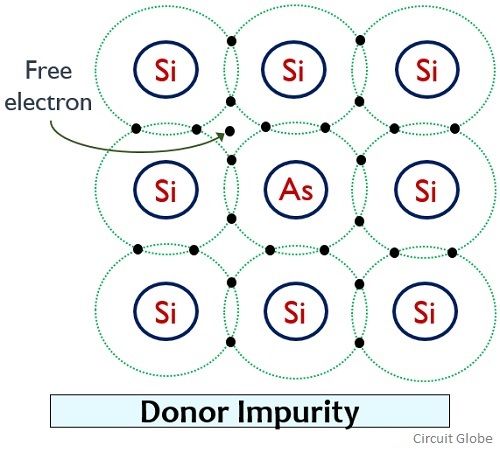
Consider that a pentavalent impurity Arsenic (As) is doped into a pure silicon structure.
As we know that arsenic has 5 electrons present in its valence shell. So, 4 electrons of arsenic form 4 covalent bonds with 4 electrons of neighbouring silicon atom as shown below:
But as we can see that here an extra electron is present. This extra weakly bonded electron freely flows around the crystal even at room temperature. The movement of this free charge inside the crystal generates current. Thus the excess electron here is known as charge carrier.
Definition of Acceptor Impurity
A dopant with 3 electrons in its valence shell, when doped with a semiconductor to raise its conductivity, is known as an acceptor impurity. It has the ability by which it can accept an electron from neighbouring atom as it has a vacancy of electron. Thus is called acceptor impurity. So, the presence of excess positive charge forms the p-type region. Thus acceptor impurity is used to form p-type semiconductors.
Group III elements are known as donor impurity because these elements consist of 3 electrons in the valence shell. Thus is known as trivalent impurity. Elements like boron, aluminium, indium and gallium are examples of trivalent impurity.
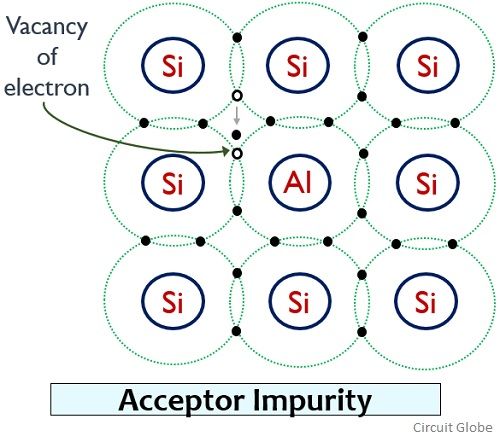
Consider an aluminium atom is doped in a pure crystal of silicon:
We know that aluminium atom consists of 3 electrons in the outermost shell. So, in this case, 3 electrons of aluminium form 3 covalent bonds with neighbouring silicon atom. But an incomplete bond exists because a vacancy of electron is present in the structure. This indicates the presence of excess positive charge (i.e., hole).
So, to fill the vacancy of that electron and complete the covalent bond, an electron is freed from neighbouring silicon atom at room temperature. This electron occupies the vacant place in the crystal thereby leaving the vacancy of an electron at the other place. In this way here a positive charge is moving in order to fill the vacant place.
Thus it is said that the movement of free charge creates current.
Key Differences Between Donor and Acceptor Impurities
- Donor impurities give its excess electrons present in its outermost shell to the other atom of the crystal structure. While acceptor impurity when added to a semiconductor then it accepts the charge from the neighbouring atom of the crystal structure.
- Donor impurity atom consists of a total of 5 electrons in its valence shell. While acceptor impurity atom consists of 3 electrons in its valence shell.
- Group V elements of the periodic table are considered donor impurity due to the presence of extra electron. However, group III elements of the periodic table are considered as acceptor impurity due to the presence of less number of electrons in the valence shell.
- Donor impurities are also known as n-type impurity. As against acceptor impurities are secondly known as a p-type impurity.
- Elements like phosphorus, antimony, bismuth, arsenic etc. are donor impurities. While boron, gallium, aluminium etc. are acceptor impurity atoms.
Conclusion
So, from this discussion, we can conclude that impurity is added in order to enhance the conductivity of the semiconductor. However, the type of added impurity leads to cause variation in the type of charge carriers responsible for conduction.

Thank you for the good information